The purpose of the thermos bowl is to prevent heat transfer between the internal liquid and the external environment—that’s how the Stanley cup and thermos work, for example. So, whether you want to keep your coffee hot for longer, in an area with a cooler temperature, or to keep your beer cold, on a typical summer day, a thermos flask is appropriate.
In this context, in order to understand how the Stanley cup and thermal containers of other brands work, it is necessary to take into account some concepts about heat diffusion. First, it is worth remembering that “heat” is a form of energy (thermal energy) that spontaneously flows from an object of higher temperature to another object of lower temperature. This transfer can occur in three ways: by conduction, convection and radiation.
Three ways to spread heat
Conduction occurs in the presence of the substance, when direct contact occurs. When exposing a metal object, for example, to a heat source, the molecules that make up the exposed section will be irritated by the incoming energy. In the case of continuity, the excitation of the particles of one section causes those in the next section to be excited as well. In this way, energy is transmitted along the element. If you touch the hot object, “heat” will also flow to your hand, due to direct contact.
Pregnancy also occurs in the presence of the substance. When you turn on an electric air heater inside a room, for example, the air at the bottom of the room will be heated. Because it is less dense and lighter, hot air rises. As warm air rises, cooler air (which is denser and heavier) near the ceiling moves downward. This process continues until the room is gradually heated up.
On the other hand, radiation, in addition to the displacement of energy in the center of matter, through particles, also allows this propagation in a vacuum, through electromagnetic waves. Infrared radiation, for example, arises from molecular vibration. When the agitation is excessive, some of the energy is converted into light.
Thermal container components
Looking at the forms of heat diffusion, it is possible to understand how the Stanley cup and other thermos flasks work. Most versions have an inner and outer compartment of plastic or metal, separated by two layers of glass with a space in between. The glass is covered with a reflective layer.
In some containers, instead of glass, there are two layers of stainless steel between which there is a vacuum and a reflective layer. Usually, an airtight, screw-on cap is added to the top of the bottle. look at the picture:
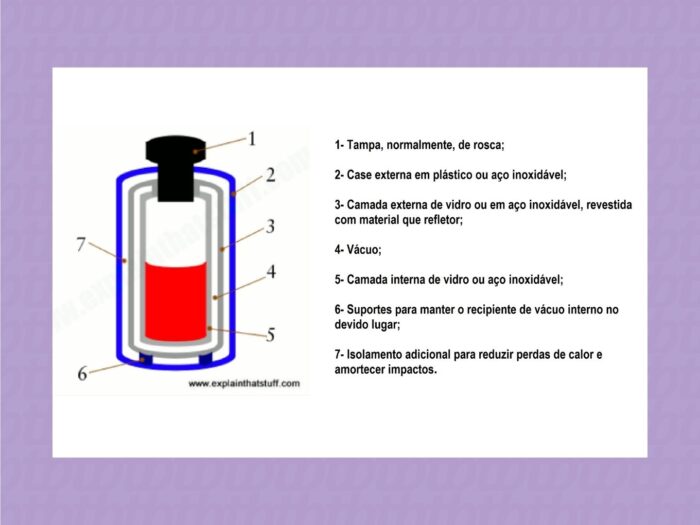
Therefore, the components of the heat pot prevent almost all forms of heat transfer. The vacuum prevents conduction and the cap prevents air from entering or leaving the flask, preventing convection. Also, when the radiation tries to make the liquid hot, the shell reflects it back. With this said, the drink can stay hot for hours.
Of course, the mechanism also keeps the brew cold for a few hours, as promised by Stanley’s thermos beer glass and Philco’s PTH01B and PTH01P models. Just as heat is temporarily prevented from escaping, heat cannot penetrate the vacuum flask by conduction. The cover prevents convection and reflects radiation.
With information from: Explain those things ¹, Explain those things ², Science ABC And the Brazil School

“Hardcore beer fanatic. Falls down a lot. Professional coffee fan. Music ninja.”





More Stories
The law allows children and adolescents to visit parents in the hospital.
Scientists pave the way for the emergence of a new element in the periodic table | World and Science
Can dengue cause hair loss? Expert explains how the disease affects hair