However, on Tuesday, a UK test program to find out how the Oxford / Astrogenogen vaccine works in children was suspended.
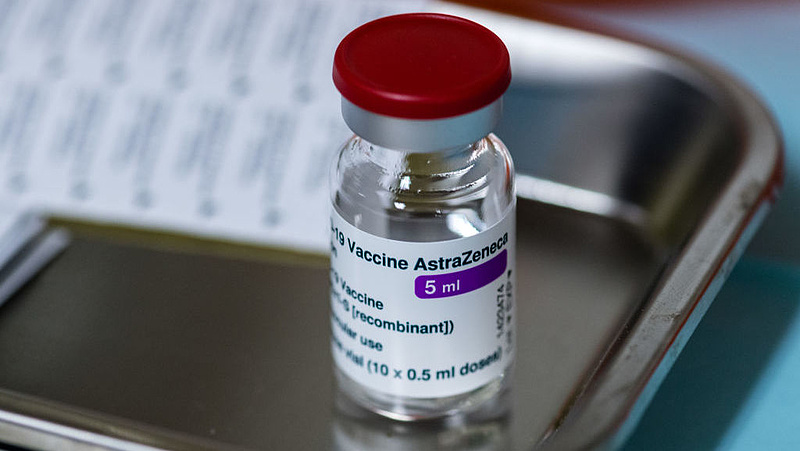
In anticipation of Nadeem Jahavi’s report on Tuesday, Marco Gavaleri, head of the European Pharmaceuticals Agency’s Vaccine Strategy Office, told the Italian newspaper on Tuesday that the Oxford / Astrogeneka vaccine has been clotting in recent weeks.
However, it is not yet known what triggered these reactions, the police added.
Speaking to the BBC British Public Service Radio on Tuesday, Jahawi said the UK Oversight Commission was closely following any reports of adverse reactions following the use of the vaccine.
In an earlier day call, MHRA CEO June Raine recalled that when it was announced that a local vaccination center could be visited, everyone was asked to continue paying for the vaccine. With nearly 20 million doses of the Oxford / Astrogeneca vaccine given in the UK so far, Zahawi said, “things need to be put in context.” Experts estimate that between the start of the UK vaccination campaign on December 8 and the end of February, the Oxford / Astrogeneneka vaccine and the Pfizer / Bioentech vaccine saved about 6,300 lives in the UK.
A recent study by the MHRA found that by March 24, more than 18 million of the Oxford / Astrogenogen vaccine had been administered in the UK, and that thirty of those vaccinated had blood clots, most of which are rare forms of cerebral thrombosis. Seven patients died.
In recent communications to the results of the study the authority emphasized that there is still no evidence of a causal link between the use of Oxford / Astrogeneka and cases of thrombosis, but the benefits of using this vaccine were far greater than the potential risks.
The Oxford / Astrogeneca vaccine and the Pfizer / Biotech vaccine are currently used in the UK.
The UK government aims to make the first dose of the corona virus vaccine available to all British adults by the end of July.
Andrew Pollard, director of the vaccine development committee at Oxford University, announced Tuesday night: Suspended A pilot vaccination program to find out what level of immunity the Oxford / Astrogenogen vaccine provides to 6-17 year olds.
The trial program began in February with 300 participants.
Speaking to BBC Radio, Professor Pollard stressed that no safety concerns had been raised about the experimental medical vaccination program for children. However, he said that before continuing the testing program in children, program management professionals would like to wait for the results of a study initiated by MHRA in adults.

“Internet evangelist. Writer. Hardcore alcoholaholic. Tv lover. Extreme reader. Coffee junkie. Falls down a lot.”




More Stories
Kamala has warned that democracy in America will be in danger if Trump wins
The world’s rarest donkey has been born at a zoo in the United Kingdom; Watch the video
Senators travel to America in search of best practices…