The new term approved by the regulator changes from four and a half months to six months, under specified storage conditions, from 2°C to 8°C.
By Brazil Agency – Brasilia
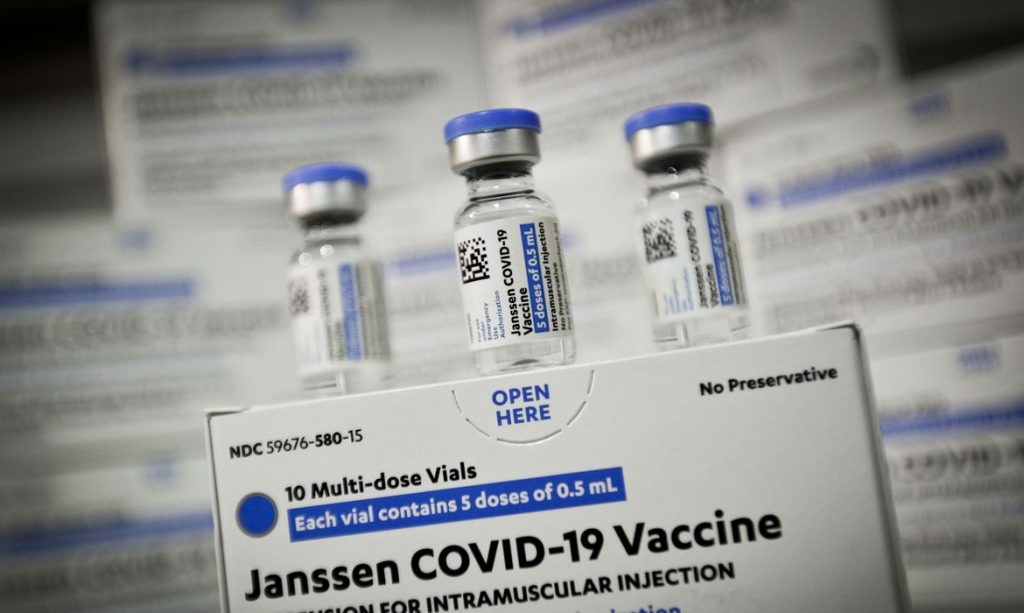
The collective board of the National Health Surveillance Agency (Anvisa) has unanimously agreed to extend the shelf life of Janssen (Johnson & Johnson) vaccine against covid-19. The term is extended from four and a half to six months, under storage conditions from 2 ° C to 8 ° C.
Janssen-Cilag Farmacêutica requested, on 15 September, a change of the validity period of the temporary authorization for emergency use, on a trial basis, of the fortifying agent.
– Continue after announcement –
According to Anfisa, approval was based on a careful evaluation of the quality data of studies that showed the vaccine remained stable for six months.
In Brazil, the Janssen vaccine has been licensed for emergency use since March 31 of this year. The vaccine is the only one approved by Anvisa in a single dose, and when stored between -25°C and -15°C, it has a shelf life of 24 months from the date of manufacture.
Related

“Hardcore beer fanatic. Falls down a lot. Professional coffee fan. Music ninja.”




More Stories
Sabesp Receives Brazil Innovation Value Award 2024 • PortalR3
Total formal job creation reached 201.7 thousand in June, up 29.6% | Economy
10,000 Brazilian Reals are waiting for you at Nubank? Find out who can get this money!