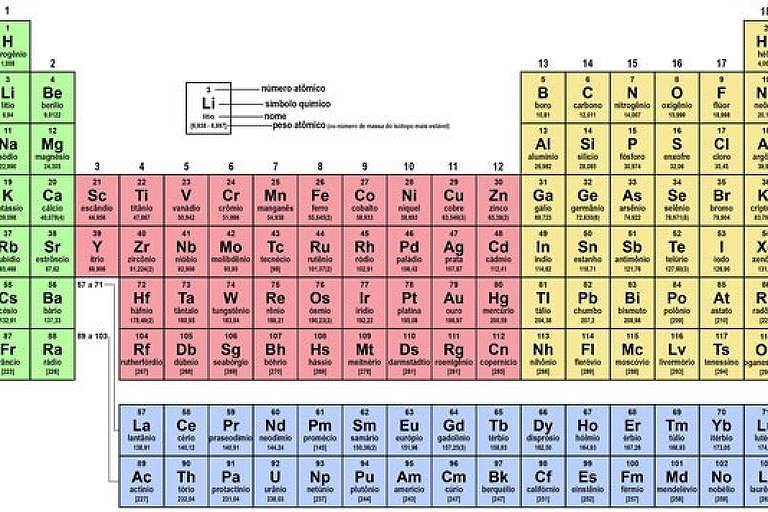
This year, I gave myself the mathematics of the periodic table for my birthday. The periodic table of the elements is one of the most famous discoveries in science. She left the laboratories and was regularly represented in objects of pop art: T-shirts, mugs, towels and even shower curtains. How can I not be intrigued by the mathematics behind the “advertising genius” of chemistry?
The problem goes back to antiquity. The Greeks Empedocles (circa 494 BC – 434 BC) believed that every substance is a mixture of four “elements”: earth, water, air, and fire. More than two thousand years later, Antoine Lavoisier (1743-1794) listed 33 elements in a “Preliminary Treatise on Chemistry” published in 1789. In the following century, chemists sought to understand and classify them.
John Dalton (1766-1844) argued that chemical elements consist of indivisible particles, which he called atoms. Other substances will be made up of molecules made up of atoms of different elements.
Johann Dobereiner (1780-1849) identified a “triad” of elements with similar properties, such as lithium, sodium, and potassium. Auguste Keccoli (1829-1896) noted that carbon has a valency of four, that is, its atoms combine with four hydrogen atoms. The elements in the same trinity have the same valency.
Alexandre-Émile de Chancourtois (1820–1886) was the first to suggest that when elements are listed in order of increasing weight of their atoms, their chemical properties repeat periodically. Lothar Meyer (1830-1895) noted inconsistencies in this law, one of which sought to explain it by predicting the presence of an unknown element, with atomic weight of 73, between silicon and tin. This element was identified in the laboratory more than twenty years later and named germanium.
John Newlands (1837-1898) went further in understanding the law of periodicity, noting that physical and chemical properties repeat in cycles of eight elements. It was believed that this is related to the octaves of music, but his “law of octaves” was ridiculed by contemporaries.
The transformation occurred in the years 1869-1871, with the publication of Tables of the Elements by Lothar Meyer, and above all the publication of the Russian chemist Dmitriy Mendeleev (1834-1907). We will continue next week.
Current link: Did you like this column? A subscriber can issue five free visits to any link per day. Just click the blue F button below.

“Hardcore beer fanatic. Falls down a lot. Professional coffee fan. Music ninja.”




More Stories
The law allows children and adolescents to visit parents in the hospital.
Scientists pave the way for the emergence of a new element in the periodic table | World and Science
Can dengue cause hair loss? Expert explains how the disease affects hair